 Dr. Taylor Wang (on right) discusses diabetes cure and bio-artificial pancreas technology with NASA Administrator Charles Bolden.
Dr. Taylor Wang (on right) discusses diabetes cure and bio-artificial pancreas technology with NASA Administrator Charles Bolden.
US Patent Application for "Tapered Channel" Capsule
New Encapsulation Advance to Yield Islet Longevity Breakthrough and Long-term Functional Cure.
June 28, 2014 — A Statement by Encapsulife President, Tom Gibson: “Based on observations and data from Dr. Taylor Wang’s successful primate trials with Dr. James Markmann of Harvard Medical School, Encapsulife has filed for U.S. patent protection for a new invention related to advanced living-cell encapsulation technologies. This new invention yields dramatically improved capsule/insulin production-and-release performance in diabetic recipients by creating a new system of pore structures in our multilayer capsule system. This, in turn, means fewer encapsulated islets are required to achieve insulin production goals to reverse diabetes. Fewer encapsulated islets in a patch system means less competition among islets for nutrients, which, in turn, leads to substantially improved longevity. Various informal estimates are three years or more of sustained islet functionality without decline. If there is decline, a booster patch would be the simple clinical treatment solution. Our focus now is preparation for FDA human trials – and getting this technology into the global healthcare system.”
US Patent Issued to Encapsulife
Patent for World’s First Living Cell Bio-Artificial Pancreas that Does Not Require Immunosuppression Drugs to Prevent Rejection in Diabetics.
March 18, 2014 — A Statement by Encapsulife President, Tom Gibson: “The U.S. Patent and Trademark Office (USPTO) issued the world’s first patent – US 8,673,294 – for a living-cell, bio-artificial organ to Dr. Taylor Wang, Encapsulife’s Founder. We are delighted. The USPTO patent applies broadly to endocrine disorders and neurological disorders, more narrowly to pancreatic disorders (diabetes), and specifically validates the efficacy of Dr. Wang’s technologies to automatically, biologically, reverse diabetes. Notwithstanding the broad ‘platform’ applications of the ‘Wang Patch,’ we are laser-beam focused on getting this invention into human trials and to diabetes suffers as quickly as resources permit.”
Wang Reports Successes in Primate Trials
JDRF-Helmsley Islet-Implant Consortium Receives Technical Briefing on Successful Primate Trials Using Living-cell Encapsulated Islets in “Patch” Configuration, Where No Immunosuppression is Required.
October 21, 2013 — A Statement by Encapsulife Chairman, Dr. Taylor Wang: “Although much of our data remains embargoed, I was pleased to report our successful primate trials working in collaboration with Dr. James Markmann. We will now proceed as quickly as possible to do additional longevity studies and proceed to human trials. It was flattering to receive the congratulations from so many of our consortia peers on our unique achievements.”
U.S. Patent and Trademark Office Issues Notice of Allowance of Encapsulife’s “Bio-Artificial Pancreas-Patch” Patent Claims
October 21, 2013 — A Statement by Encapsulife President, Tom Gibson: “Having filed for this patent five years ago, it is remarkable that we received the Patent Office’s Notice of Allowance in the same week that Dr. Taylor Wang reports the successes of his Bio-Artificial Pancreas to a conference of world experts on islet transplantation. Given the various other conference presentations, we believe no other research group has achieved such dramatic milestones toward curing diabetes (reversing diabetes both in canines and primates without immunosuppression drugs).
We must now move urgently to get this technology into human trials and to victims of diabetes. Ironically, implanting the subcutaneous “patch” should prove simpler and more efficacious in humans than in small primates. Non-human primates require four to eight times more insulin than humans per kilogram body weight – and primates cannot be restrained or told to ‘take it easy for a few days’ after the implant procedure, to enable the patch implants to settle-in. Our development efforts will now go into high gear.”
October 19, 2013 — A Statement by Geoffrey Mason, patent expert and CEO of FastPatentPartner: “It is unusual for the Patent and Trademark Office to allow a claim with an efficacy statement such as the one in the Encapsulife’s Letter of Acceptance. It shows the PTO believes the invention will work.”
February, 2012 — A Statement by Encapsulife Chairman, Dr. Taylor Wang: “We are now entering the third year of an important and successful three-year study. Our recent success in diabetes management in NHP non-human primates is very encouraging. Using our Bio-artificial pancreas ‘patch’ technology, we have demonstrated long-term islet in vivo survival. We should be in the position to address FDA requirements for human clinical trials this year.”
Encapsulife Files Patent Application for Bio-Artifical Pancreas "Patch"
Patch Technology to be Tested in Primate Trials
January, 2010 — Encapsulife has filed a patent application with the U.S. patent and Trademark Office related to the invention of a bio-artificial pancreas "patch." The invention successfully holds large numbers of encapsulated islet cells in formation while maintaining sufficient mass-transport capabilities to sustain functionality of the islet cells in-vivo and the production of insulin. No harmful immunosuppression drugs are required to prevent rejection by the host immune system.
Encapsulife Publishes New Peer-Reviewed Research
Immunoisolation of Transplanted Beta Cells Reverses Diabetes in Large Animal Trials Without Immunosuppression
 A bio-artificial pancreas “patch” with encapsulated islet cells, ready for simple subcutaneous transplant. No immunosuppression drugs are required to prevent rejection.
A bio-artificial pancreas “patch” with encapsulated islet cells, ready for simple subcutaneous transplant. No immunosuppression drugs are required to prevent rejection.
 A single encapsulated cluster of islet cells (very high magnification).
A single encapsulated cluster of islet cells (very high magnification).
Utilizing insights from compound droplet experiments performed in the microgravity of NASA Shuttle Mission STS-51-B, Encapsulife founder, Dr. Taylor Wang, has developed an immunoisolation encapsulation system that protects cellular transplants, and sustains cell function — without immunosuppression drugs and their resulting negative side effects.
This novel immunoisolation system is a multi-component, multi-membrane capsule that allows independent optimization of all capsule design parameters ensuring reproducible functions in large animals and humans. Results of Encapsulife's successful large animal trials, have recently been published in peer-reviewed research in Transplantation Journal. In this landmark research, encapsulated canine pancreatic islets were transplanted into dogs rendered diabetic by total pancreatectomy. No immunosuppression or anti-inflammatory therapy was used.
The allotransplantations of encapsulated islets were well tolerated and biocompatible, and normalized fasting blood glucose levels in all of 9 dogs, were achieved for over two hundred days, with a single transplantation. Re-transplantation of encapsulated islets — a "booster" — was effective in providing glycemic control beyond the initial 200 days.
Other immunoisolation systems have been tested in large animal models and one in humans; many of those experiments were performed in spontaneous diabetic subjects or utilized immunosuppressive agents. Many of these experiments were not or cannot be reproduced.
Encapsulife's advancements in living cell transplantation are intended to lead to alternative approaches for islet transplantation treatment for diabetic patients. Encapsuife collaborations are now under way with leading research groups to advance this science to clinical human trials. This approach may also benefit patients suffering from other hormone deficiency diseases — including liver disease and Parkinson's disease.
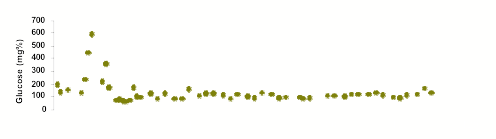
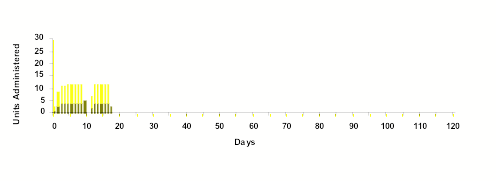
Notes on Graphic
Top Panel: Data points indicate the venous plasma glucose concentrations collected 12-18 hours following a meal. A level of between 80 and 120 is deemed "normal."
Lower Panel: Indicates the daily dosage of subcutaneous porcine insulin administered, in an attempt to maintain normal plasma glucose concentrations (blood sugar). Two types of insulin are used in combination. Upper portion of bar indicates NPH insulin and lower portion of bar indicates Regular insulin. After Day 18 — NO Insulin Injections were administered.
The data associated with Encapsulife's research remains part of Encapsulife's patented intellectual property and portfolio of trade secrets. However, the data below, which has been reviewed by scientific peers and research supporters — is representative of diabetes reversal in one of the 9 canine subjects in Encapsulife's recently completed large animal trials.
- On Day 1, the subject's pancreas was removed, insulin production ceased, Type 1 diabetes was therefore induced, and supplemental insulin injections ("units" on vertical axis) were required to stabilize blood glucose (BG) levels and preserve life in the subject.
- On Day 10, insulin was withheld for 1 day, the diabetes BG returned to ~ 600 immediately.
- On Day 18, after partial glucose stability was recovered in the subject through insulin injections, encapsulated islets were transplanted into the subject. No immunosuppressive drugs were used.
- Days 19 through 120, transplantation was well-tolerated with minimal inflammatory response. At 120 Days, glucose levels remained normal. (HbA1c=6.0)
